Formula for an Ionic Compound Made of Magnesium and Sulfur
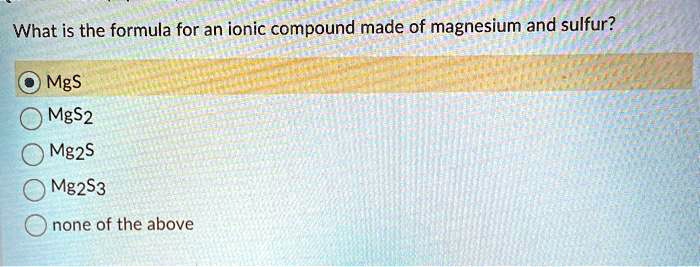
It will take one -2 sulfide ion to balance one 2 magnesium ion forming a magnesium sulfide molecule of MgS. A MgS b MgS2 c Mg2S d Mg2S3 e None of the above.

Solved What Is The Formula For An Ionic Compound Made Of Chegg Com
Magnesium sulfide represented by the chemical formula MgS that bears the IUPAC name sulfanylidenemagnesium is a white crystalline inorganic compound that is moderately soluble in water and acid.
. What is the formula for an ionic compound made of magnesium and sulfur. In order to bond ionically the charges must be equal and opposite. In order to bond ionically the charges must be equal.
MgS MgS_2 Mg_2S Mg_2S_ What are the coefficients for the following reaction when it is properly balanced. It needs two potassium atom to join with sulfur to form and ionic compound. The electrostatic force of attraction will then bring the magnesium cations and the sulfur anions together an ionic bond is formed.
In order to bond ionically the charges must be equal and opposite. What is the formula for an ionic compound made of magnesium and sulfur. A a Mg2S2 Mg2S2 MgS a a.
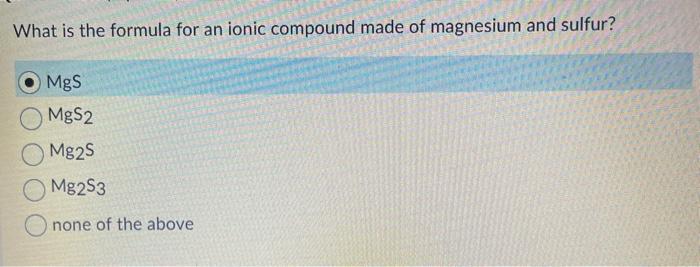
To determine the formula locate both. What is the formula for an ionic compound made of barium and nitrogen. O MgS MgS2 Mg2S Mg2S3 Onone of the above.
Magnesium Sulfide has a formula of MgS. It will take one -2 sulfide ion to balance one 2 magnesium ion forming a magnesium sulfide molecule of MgS. Does sulfur and potassium form an ionic compound.
The Lewis theory predicts that the formula for a compound of magnesium and sulfur is MgS2. This is an ionic bond and is the basis of the ionic compound formed as the differently charged ions are held together by their opposite. What is the formula for an ionic compound made of magnesium and sulfur.
In order to bond ionically the charges must be equal and opposite. MgS Mg2S3 Mg2S MgS2 none of the above. It will take one -2 sulfide ion to balance one 2 magnesium ion forming a magnesium sulfide molecule of MgS.
What is the formula for an ionic compound made of magnesium and sulfur A MgS B from CHEM 113 at Northwest Missouri State University. What is the formula for an ionic compound made of magnesium and sulfur. What is the formula for an ionic compound made of magnesium and sulfur group of answer choices.
The product is magnesium sulfide with formula MgS. What is the formula for an ionic compound made of magnesium and sulfur. What is the formula for an ionic compound made of magnesium and sulfur.
4 rows Magnesium Sulfide has a formula of MgS. The difference in charge between the two 1 potassium ions and the 2- sulfur ion allows electrostatic attraction between the sulfur ions and potassium ions. Chemistry questions and answers.
What is the formula for the ionic compound of magnesium and sulfur. It is an ionic compound of magnesium and sulfur. Magnesium Sulfide has a formula of MgS.
Magnesium Sulfide has a formula of MgS. The formula for barium nitride is Ba3 N2. What is the ionic formula for magnesium and sulfur.

Solved What Is The Formula For An Ionic Compound Made Of Magnesium And Sulfur Mgs Mgs2 Mg2s Mg2s3 None Of The Above

What Is The Formula For An Ionic Compound Made Of Magnesium And Sulfur Lisbdnet Com
Solved What Is The Formula For An Ionic Compound Made Of Chegg Com
Comments
Post a Comment